Adiabatic cooling is a term rooted in thermodynamics, which deals with the behavior and properties of energy and matter. The word “adiabatic” originates in Greek, meaning “impassable.” This directly reflects the principle that in an adiabatic process, there is no energy exchange in the form of heat with the surroundings. It’s all about how a substance, primarily a gas, changes its temperature when it expands without gaining or losing heat.
What is Adiabatic Cooling?
Adiabatic cooling represents the process of health reduction because of the air pressure change during volume expansion. It occurs when a gas expands without exchanging heat with its surroundings, decreasing its temperature. It’s a fundamental thermodynamic process in which the gas’s internal energy is used to work on the surroundings, resulting in cooling.
Understanding the Basics
- Adiabatic vs. Isothermal: These are two contrasting thermodynamic processes. While adiabatic processes see a change in temperature without heat exchange, isothermal processes occur at a constant temperature, regardless of the changes within the system.
- Energy Conservation: One of physics’s fundamental laws is energy conservation. It states that energy can’t be created or destroyed; it can only change forms. So, when a gas expands and cools in an adiabatic process, the power isn’t lost; instead, it’s converted into work done on the surroundings.
Mechanisms at Play in Adiabatic Cooling
- Expansion Leading to Cooling: Imagine holding a balloon filled with air. If you suddenly release the air, you’ll notice the escaping air is more relaxed. This is adiabatic cooling in action. As the gas expands, it uses its internal energy to work on its surroundings, leading to a drop in its temperature.
- The Flip Side – Compression and Heating: The opposite of expansion in adiabatic processes is compression. When a gas is compressed, work is done on it. This increases its internal energy, and as a result, its temperature goes up. This is known as adiabatic heating.
Applications in the Real World
- Meteorological Phenomena: Our weather patterns owe a lot to adiabatic cooling. As moist air rises in the atmosphere, it expands due to decreasing atmospheric pressure. This expansion is an adiabatic process and leads to cooling. Condensation occurs once the temperature drops enough for the air to reach its dew point, leading to cloud formation and potentially rain.
- In the World of Refrigeration: Rapid cooling technologies sometimes leverage adiabatic processes. Allowing a refrigerant to expand quickly cools down, which cools the surrounding area or substance.
- Braking Systems in Large Vehicles: When large vehicles like trucks apply their brakes, the air in the brake system is compressed rapidly. This is an adiabatic process, and the compressed air heats up. This is why the design of these systems is crucial to prevent overheating.
- The Vastness of Space: Adiabatic processes aren’t limited to our planet. They are vital to the study of stars and celestial bodies. As stars evolve, they undergo expansion and contraction, and understanding adiabatic processes helps astronomers predict and explain these behaviors.
Adiabatic Equations
In thermodynamics, equations help quantify and predict the behavior of substances. For gases undergoing an adiabatic process, there exists a relationship between pressure and volume, often expressed with terms like “constant” and “gamma” (a heat capacity ratio). These equations can be used to determine the end state of a gas after it has undergone an adiabatic process, given its initial conditions.
Limitations and Realities
It’s essential to note that while the concept of an adiabatic process is valuable in theoretical scenarios, achieving a perfectly adiabatic process in the real world is challenging. Due to inherent inefficiencies and external factors, there’s always some degree of heat exchange, however minimal. Yet, understanding the adiabatic ideal gives us benchmarks and guides in numerous scientific and industrial applications.
What causes adiabatic cooling?
Adiabatic cooling causes rapid gas expansion. So, we defined adiabatic cooling as heat reduction because of the air pressure change during volume expansion.
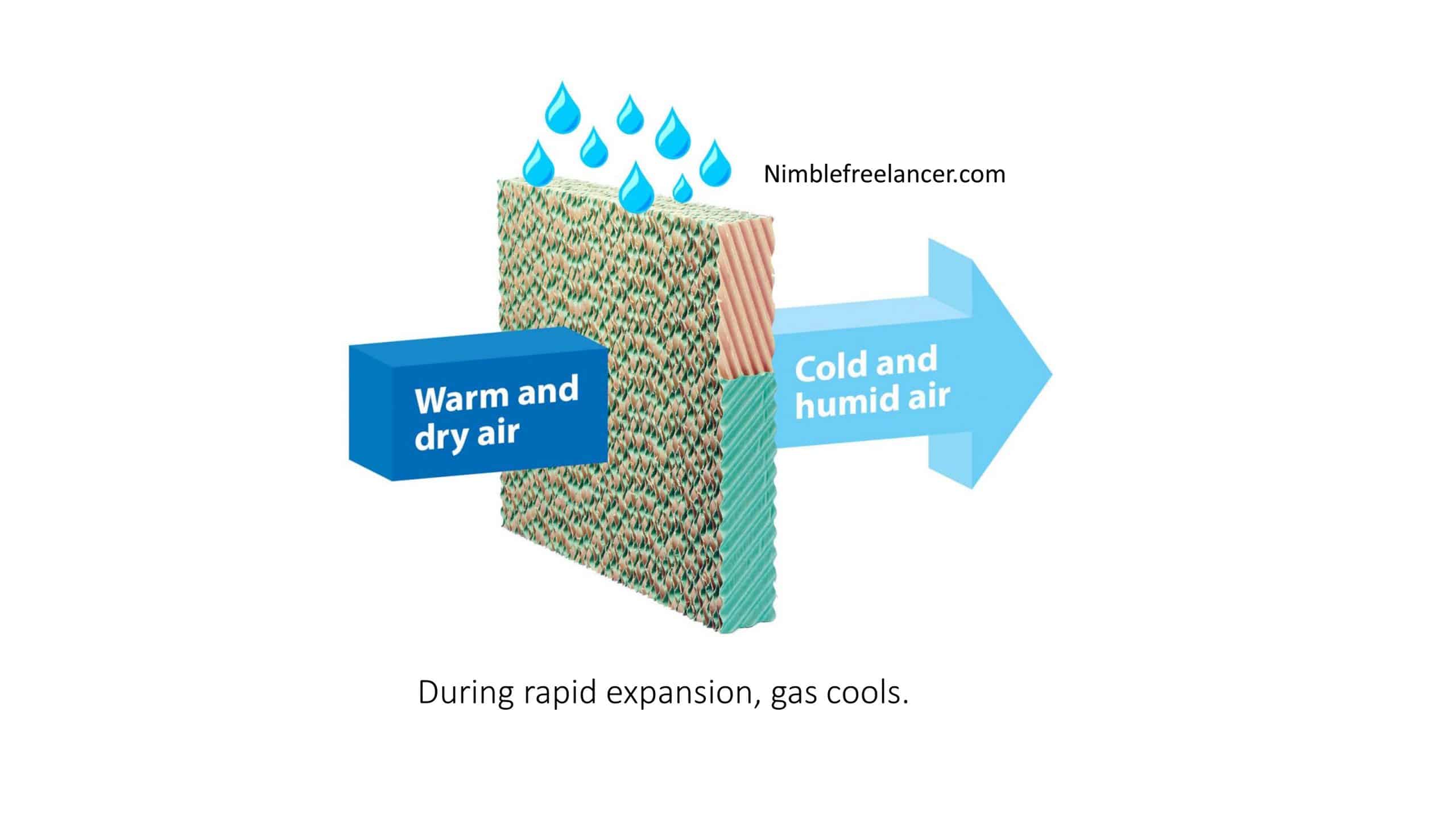
Adiabatic cooling is utilized in evaporative coolers. An evaporative cooler is an enormous fan that draws warm air through water-soaked cushions. As the water in the cushions vanishes, the air is chilled and pushed out to the room. The temperature can be constrained by changing the cooler’s wind stream.
In nature, adiabatic cooling is frequently connected with rising. As seen with cloud developments, warm air mass grows and becomes less thick. Being less dense, it is lighter and transcends a higher-pressure air mass. Having arrived at zones with less dense air, it further extends, losing energy acquired and cooling as it does. When the cooling air crosses the dew point, dampness is noticeable all around aggregates as mists. With enough dampness and cooling comes precipitation. The standards of adiabatic cooling are likewise applied to build moistness in offices.
Adiabatic Cooling in Nature video:
Then again, adiabatic warming outcomes when a cooler, less thick air mass sinks and expands in temperature because the compressed particles get upset, vibrate, and expand heat.
Adiabatic cooling costs have been significantly reduced. By using a kinetic heat transfer model, the values for the steady-state and cyclotron modes have been increased to 1.6 and 1.65 electronvolts, respectively. Another proposed improvement is to replace the cryostat with a turbulent heat exchanger. A magnetohydrodynamic ramjet is a theoretical magnetic ramjet that converts its kinetic energy into usable power through magnetohydrodynamic (MHD) heat flow.
Adiabatic cooling is essential because:
- Natural Evaporation Process: This process utilizes the inherent properties of water evaporation to achieve cooling, making it more sustainable and environmentally friendly.
- Superiority Over Traditional Systems: Offers a more efficient cooling alternative than conventional methods, often delivering more consistent and effective results.
- Cost-Efficiency: The operational and maintenance expenses associated with adiabatic cooling can be up to 80% less than traditional cooling systems, providing significant savings in the long run.
- Energy Conservation: Adiabatic cooling systems often consume less power by relying on natural processes and being more efficient, reducing energy bills.
- Environmental Impact: The reduced energy consumption directly translates to fewer CO2 emissions, making adiabatic cooling a more eco-friendly option.
Adiabatic cooling works according to the standards of thermodynamics, where energy (heat) is moved starting with one medium and then onto the next as “work” without a genuine mass trade. The adiabatic cooling measure happens when a decrease in the pressing factor inside a framework causes a volume development, bringing about “work” on the general climate.
A commonplace adiabatic cooling framework pulls air from the outer climate, decreases its temperature by dissipating water in its quality, and takes care of the cooled air to a warmth exchanger. The warm exchanger dispenses with heat energy from the related cycle/hardware and moves it to the cool encompassing air. When warmed, the temperature of the circling air is brought down by vanishing before another cooling cycle.
Consistently, gigantic amounts of new water are devoured using conventional evaporative frameworks for the warmth dispersal of modern cycles (cooling towers). The expanding lack of water assets, the trouble in discovering them, and the continually expanding costs push the business towards this innovation, ready to set aside 95% of water: adiabatic cooling. This innovation also gives more prominent heat move proficiency, decreased upkeep, and the all-out shortfall of dirtying synthetic specialists.
Conclusion
Adiabatic cooling is more than just a fascinating scientific concept; it’s a principle that governs many natural and technological processes around us. Understanding its mechanics, principles, and applications gives us deeper insights into our universe’s complex dance of energy and matter. Whether it’s predicting tomorrow’s weather or designing the next generation of refrigeration systems, adiabatic cooling remains a cornerstone concept in the vast world of thermodynamics.