At first glance, adiabatic and evaporative cooling might seem synonymous, given that both leverage the principle of evaporative heat loss to achieve cooling. However, the terms have nuanced differences, especially regarding their technical definitions and implementations. Let’s delve into these processes to understand their differences more clearly.
Adiabatic Cooling:
- Definition: Adiabatic cooling refers to the process wherein a substance, typically a gas, changes its temperature (cools) when it expands without exchanging heat with its surroundings. The essential feature here is the absence of heat exchange.
- Mechanism: When a gas expands in an adiabatic process, it does work on its surroundings, causing its internal energy to decrease. This decrease in internal energy leads to a reduction in temperature.
- Applications: The most common example is the rising of an air parcel in the atmosphere. As it ascends, the air parcel expands due to decreasing atmospheric pressure, and if no heat is exchanged with the surroundings, it cools adiabatically.
Evaporative Cooling:
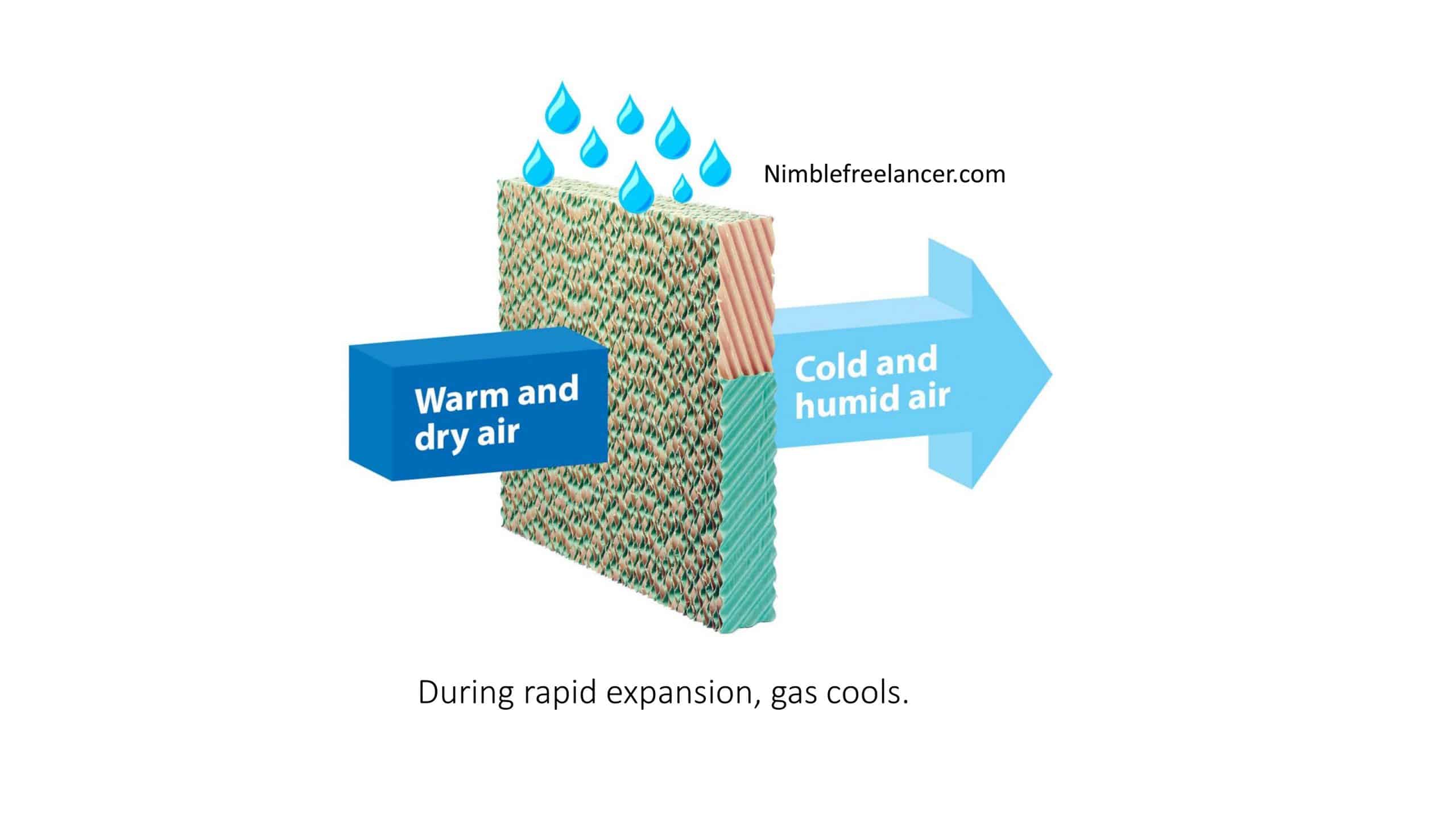
- Definition: Evaporative cooling is when a liquid (commonly water) evaporates, absorbing heat from its surroundings and reducing the ambient temperature.
- Mechanism: For a liquid to transform into a vapor, it needs energy (latent heat of vaporization). This energy is taken from the liquid itself and its immediate surroundings. As the liquid evaporates, it absorbs this necessary heat, causing the surrounding environment to cool.
- Applications: Common applications include swamp coolers or wet cooling towers, where water is exposed to air, facilitating its evaporation and cooling of the air.
Key Differences:
- Nature of Substance: Adiabatic cooling typically involves gases, whereas evaporative cooling involves the phase change of a liquid to a vapor.
- Heat Exchange: The fundamental feature of an adiabatic process is no heat exchange with the environment. In contrast, evaporative cooling inherently involves heat exchange since the heat required for evaporation is extracted from the surroundings.
- Applications: While both processes can be seen in atmospheric phenomena (like cloud formation), their technical applications differ. Adiabatic cooling is a principle used to understand processes in air parcels and general thermodynamics. In contrast, evaporative cooling is more often directly harnessed for cooling systems in buildings, industrial settings, or electronic systems.
Conclusion
While both adiabatic and evaporative cooling are natural processes that achieve a drop in temperature, they operate based on different principles and contexts. Understanding these differences is essential, especially when selecting or designing cooling systems for specific applications.